-40%
Portable LED Cold Light Source 3W - 10W for Surgical Endoscopy Endoscope Surgery
$ 55.43
- Description
- Size Guide
Description
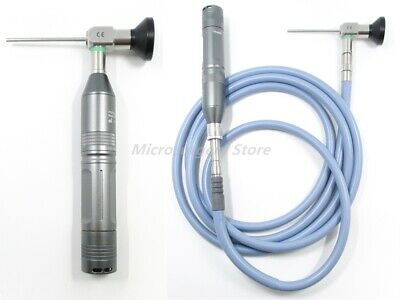
Features:The unit built-in LED bulb, the high energy conservation, the multi-transformations, the favorable environmental protection, the long life cycle. It provides the color temperatureofapprox.9500k corresponding to sunlight. The latest circuit technology provides optimum performance and functionality.
Compact and portable, the built-in lithium battery allows the Mini Handheld Cold Light Source to work more than 2 hours after being recharged. We design and manufacture it for use either with a rigid or flexible endoscope. A particularly well conceived safety feature is the automatic switchover from main light source to the spare light source in case of light source failure. Therefore the light source is always ready for emergency use and contributes to patient safety.
Technical Data
Power supply: AC110/220V±10%, 50/60Hz
Battery: Lithium battery
Bulb: LED OSRAM bulb, approx.5W
Safety standard: IEC601-1
Color temp.:approx.9,500 K
Illumination:
≥140
,
000 Lx
Life cycle of bulb: approx.1,200 h
Storage temp.: -15℃ to +50
℃
Operating temp.: 0℃ to 45℃
Relative humidity:30% to 90%
Device type: I/B
Light of flexible :φ27mm, L:145mm.
N.W.: 0.25 kg
Light of rigid endoscope :φ24mm, L:115mm.
N.W.: 0.2 kg
Packing List:
1. LED cold light source: 1 each
2. Battery Charger: 1 each
3. Rechargeable batteries: 2 each
FDA declaration :
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(CICI-China-86-18328503374)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892